Long-term efficacy of Transjugular Intrahepatic Portosystemic Shunt treatment for Budd-Chiari Syndrome
Copyright
© 2017 Upon acceptance of an article for publication in Hellenic Journal of Radiology, authors transfer copyright to the Hellenic Radiological Society but they retain the intellectual property rights including research data.
Introduction
Budd-Chiari Syndrome (BCS) is an uncommon condition caused by the obstruction of the hepatic veins and/or the hepatic portion of the inferior vena cava (IVC) resulting in a wide variety of symptoms [1]. Initially described by the British physician George Budd in 1845 [2] and later, in 1898, by the Austrian pathologist Hans Chiari [3], two major categories have been proposed: Primary BCS, defined as hepatic venous outflow obstruction originating from endoluminal venous lesion (thrombosis, webs, endophlebitis) and secondary BCS, defined as hepatic venous outflow obstruction originating from a lesion outside the venous system (tumour, abscess, cysts, and pericardial conditions). Lesions may obstruct outflow by invading the lumen or by extrinsic compression [4, 5]. Practically, when no causes of secondary obstruction are found, Budd-Chiari syndrome is regarded as primary and is further classified in two types according to the anatomical location of the venous obstruction: A “classical BCS” type in which the obstruction occurs within the hepatic vein and “hepatic vena cava BCS” which implies thrombosis of the intra/suprahepatic portion of the IVC [6]. The importance of this division lies on the different prognosis of the two types of primary BCS: The former has potentially more severe outcome than the latter which has a more chronic evolution and milder symptoms. In the western world, classical BCS is the most common form of primary BCS, whereas the most frequent cause of hepatic vein occlusion is thrombosis due to myeloproliferative diseases (thrombophilic disorders). On the contrary, in East Asian population, hepatic vena cava BCS is the most common form of the primary BCS and is mostly idiopathic or related to anatomical anomalies such as membranous obstruction [7]. The most frequent clinical feature of BCS is ascites, which is also the most common indication for TIPS in case of medical treatment failure [8].
Prevalence of BCS remains largely unknown but estimates range between 1/50,000 and 1/100,000. The natural course of the disease is highly unfavorable and although prognosis is promising in case of early diagnosis and rapid treatment initiation leading to up to 90% survival rate at 5 years, if left untreated reduced hepatic venous outflow leads to hepatic congestion and rapid fibrosis usually within 3 months, with a mortality rate of 50% in 2 years. Less than 10% of untreated patients will survive for more than 3 years. Mortality depends on the occurrence of complications such as portal vein thrombosis, IVC thrombosis or renal failure [9, 10].
In BCS management, a step-by-step approach, based on clinical presentation, time of thrombosis and liver function reserve has been adopted. In case of acute BCS anticoagulation therapy is the first line approach, maintaining INR between 2.0 and 2.5, while ascites can be treated using diuretic therapy and/or paracenteses. Fulminant BCS requires a more aggressive treatment approach, thus catheter-directed local thrombolysis combined with angioplasty [11]. Nevertheless, in case of medical therapy failure, again more invasive therapeutic options should be considered, including surgical shunts or less invasive percutaneous Interventional Radiology techniques, such as transjugular intrahepatic portosystemic shunt (TIPS). The rationale of portosystemic shunt (surgical or interventional) is the reduction of portal vein pressure and splanchnic congestion, as shunt creation allows retrograde arterial perfusion of the sinusoids of the periportal zone 1 and 2 of the liver acinus, thus reducing the hypoxic damage of the hepatocytes and leading to improvement of liver histology and function [12, 13].
Although TIPS for BCS refractory to anticoagulant therapy is recommended as a safe and effective minimal invasive treatment option, long-term outcomes reported in the literature remain scarce [14-16]. The aim of the present study is to report long-term outcomes following TIPS for symptomatic BCS refractory to medical treatment.
Materials and methods
2.1 Study design
This was a retrospective, single-centre, single-arm analysis of all patients who underwent TIPS in the Interventional Radiology department during a 13-year period, between July 2003 and December 2016, due to symptomatic BCS not responsive to medical therapy. Diagnosis of BCS was set according to the European network for vascular disorders ofthe liver (En-vie) criteria using Doppler ultrasound (DUS), computed tomography (CT), magnetic resonance imaging (MRI), or classical digital subtracted venography [17]. Model for end-stage liver disease (MELD) and BCS-TIPS prognostic index (BSC-TIPS PI=age (years) x 0.08 + bilirubin (mg/dL) x 0.16 + INR x 0.63) scores were calculated using admission data. Procedural details were also recorded.
In total, 27 consecutive patients (17 female and 10 male patients; 62.9%) with a mean age of 50.2 ± 14.9 years (range: 21- 80 years) were included in the study. BCS was related to a chronic myeloproliferative disorder in the vast majority of the patients (11/27; 40.7%), but also to hyperhomocysteinaemia (1/27; 3.7%), Churg-Strauss syndrome (1/27; 3.7%) and paroxysmal nocturnal haemoglobinuria (1/27; 3.7%). In the remaining 13 patients, no predisposing factor was recognised (idiopathic BCS). All cases were classified as “classical” BCS.
In the majority of the cases, initial clinical manifestation and main indication for TIPS was refractory ascites (25/27; 92.6%), while the remaining 2 patients presented with acute variceal bleeding not controlled by endoscopy. Portal vein thrombosis was not noted during pre-procedural imaging, while caudate lobe hypertrophy and portosystemic collaterals were present in 96.3% (26/27) and 55.5% (15/27) of the cases, respectively.
2.2 Procedure
TIPS procedure in BCS has been analytically described previously [16]. In brief, pre-procedural planning was based on triple phase liver CT with multiplanar (MPR) sagittal and coronal reconstructions and liver ultrasonography. In cases of significant quantity of ascites, a lower quadrant 8Fr external drain was placed to empty the peritoneal cavity prior to the procedure. All procedures were performed under general anaesthesia in the Interventional Radiology suite. Access was obtained from the right internal jugular vein in all cases. If no main hepatic vein was patent but a stump could be located at the site of the origin of the superior hepatic veins, the tip of the TIPS set cannula was wedged against this stump as to forward the needle toward the intrahepatic portal vein branch (20/27 cases; 70.1%). If neither patent main hepatic vein, nor stump were identified the puncture was performed directly through the intrahepatic IVC (also called Direct Intrahepatic Porto-systemic Shunt - DIPS), using bone landmarks as well as information from the MPR reconstructed images (7 cases, 29.9%). The ‘‘gun-sight’’ technique was performed in one patient. The latter technique, proposed by Haskal et al., is an alternative method of creating a porto-systemic shunt in patients with very small, angulated or occluded hepatic veins. It is meant to be used as a last resort when all other maneuvers fail. A percutaneous trans-hepatic puncture is performed and a 10 mm snare is placed within a peripheral right branch of the portal vein. Subsequently, a 25 mm snare is advanced through the trans-jugular sheath and placed within the retro-hepatic portion of the IVC. Image intensifier is then moved to an almost lateral position so that the 10 mm small portal snare is projected into the bigger 25 mm caval snare. Using fluoroscopy a new percutaneous trans-hepatic puncture is performed and a sheathed needle is advanced through the centre of the smaller and the bigger snare, which are used as landmarks for obtaining correct needle orientation. Once the puncture into the IVC is performed, the needle is removed and through its sheath a guide wire is advanced into the IVC, captured by the caval snare and extracted through the trans-jugular sheath [18].
Another technique used in case of multiple failed attempts to access the portal system was US-guided percutaneous trans-hepatic placement of a metallic coil within the target portal vein branch in order to obtain a fluoroscopic landmark for needle puncture (2 cases).
In five patients (5/27; 18.5%), TIPS was created using 12 mm diameter self-expandable bare metal stents post-dilated to 10 mm (stent median number=2). In the remaining 23 patients, e-polytetrafluoroethylene stent grafts (Viatorr Endoprosthesis; WL Gore & Associates, Flagstaff, AZ, USA) were used. In 5/23 cases (21.7%), an additional stent graft (Fluency® Plus Endovascular Stent Graft, BARD Peripheral Vascular, Tempe, AZ, USA) was used as to achieve a satisfactory full stent coverage up to the origin of the IVC. Stent-grafts were 10 mm (17/23; 78.3%) and 12 mm (5/23; 21.7%) in diameter and were post-dilated with 10 mm balloon catheters as to achieve a portosystemic pressure gradient <12 mm Hg. In all secondary procedures, only stent-grafts were used. Lifelong anticoagulation was prescribed.
2.3 Outcomes, definitions and follow up
Primary outcome measure was patient orthotopic liver transplant (OLT)-free survival. Secondary outcome measures were technical success defined as successful creation of a patent TIPS at completion angiogram and a porto-systemic gradient <12 mm Hg, clinical success defined complete elimination of initial symptoms; primary patency defined as uninterrupted patent TIPS without >50% stenosisaccording to international DUS criteria [19] and/or additional intervention; re-intervention free interval defined as the time period without clinically driven re-intervention due to TIPS dysfunction (symptoms relapse related to a significant stenosis or occlusion of the primary TIPS confirmed by DSV), as well as the identification of possible factors influencing outcomes. Procedure-related complications were also recorded.
Postoperative follow-up included clinical evaluation, laboratory studies and DUS imaging on an outpatient basis at 1-, 3- and 6-month intervals, and yearly thereafter. TIPS dysfunction initially detected by DUS was always further evaluated by selective DSV of the shunt.
2.4 Statistical analysis
Discrete variables are given as counts and percentages. Continuous variables originating from normal distributions according to the Kolmogorov-Smirnov goodness-of-fit test normality test are reported as means ± standard error (SE), otherwise as medians and interquartile ranges (i.e., between the 25th and 75th percentiles) in parentheses. Kaplan-Meier life-table analysis was used for estimation of the primary endpoint, as well as estimation of primary patency and reintervention-free interval rates.
Univariate subgroup analysis was performed as to identify factors influencing outcomes. Dependent variables were patient age, MELD score >18 and stent type (self-expandable bare metal or covered stent). The covariates were transplant-free survival and primary patency. Results are expressed as hazard ratios and 95% confidence intervals (CIs) with associated level of statistical significance. Subgroup analysis curve plots are presented only in cases of significant results. Statistical analysis was performed with use of the GraphPad PRISM statistical software package, (5th edition, San Diego, California, USA).
Results
Mean follow-up time was 44.1.1 ± 40.1 months (range 1-139). Three patients were lost to follow up 6 months after the initial procedure. Mean MELD score was 13.8 ± 4.9 (range 6-25) and mean BSC-TIPS PI was 4.9 ± 1.3 (range: 3.25 to 8.48). According to MELD score, 19 patients had intermediate prognosis (estimated 3-month mortality 6%) and 8 had good prognosis (estimated 3-month mortality 1.9%). According to BSC-TIPS PI only two patients had poor prognosis (PI>7).Technical success rate was 100%. Clinical success rate was 96.3% (26/27 procedures) as one patient did not experience symptoms relief and died of hepatic insufficiency one month following the procedure. No procedure-related death occurred. Bleeding complications were noted in 3 cases (3/27; 11.1%). Specifically, two cases of haemoperitoneum were recognised during the procedure and anotherIn one case an additional stent graft was deployed, in case of delayed bleeding trans-catheter coil embolisation was performed and in the third case surgical laparotomy and liver packing was performed for haemostasis in theater. There were also three cases (3/27; 11.1%) of hepatic encephalopathy successfully managed with standard medical therapy.According to Kaplan-Meier analysis, estimated OLT-free survival rates were 96.3%, 96.3%, 82.5% at 2, 5 and 10 years follow up, respectively (Fig. 1). Cumulative, two deaths occurred (2/27; 7.4%) within follow up period, one after one month (clinical failure) and another after 67 months follow up. Patient survival was identical to OLT-free survival, as no patient underwent OLT during follow up. Primary patency rates were 77.4%, 55.3% and 26.3% at 1, 2 and 8 years follow up, respectively (Fig. 2a).Reintervention-free interval rates were 80.4%, 57.4% and 30.8% at 1, 2 and 8 years follow up, respectively (Fig. 2b). Cumulatively, 17.5% of the patients (10/27 cases) required at least one re-intervention during follow up, while medium reintervention number in these patients was 1 (range: 1- 7). Specifically, in six patients 1 re-intervention was required, in two patients two re-interventions were required, in one patient 3 re-interventions were required and one patient underwent 7 re-interventions, for a total of 21 re-interventions. In one case (1/11 cases of shunt restenosis/ occlusion; 9.1%), although shunt occlusion was confirmed by both DUS and CT imaging, the patient remained asymptomatic throughout the follow up period and therefore no reintervention was decided. Patient MELD score and age at the time of the procedure did not influence survival. According to univariate subgroup survival analysis stent graft use was correlated with significantly better survival compared to bare metal stent (HR: 0.0045; 95% CI 0.00003 to 0.701; p=0.035) (Fig. 3a). Primary patency was also significantly superior when stent grafts were used (HR: 0.36; 95% CI 2.503 to 3.053; p=0.03) (Fig. 3b).Specifically, in patients treated with stent grafts median primary patency was 50 months vs. 15 months for bare stents. Bleeding complications were significantly superior when additional trans-hepatic access was utilised (3/3 cases; 100% vs. 0/24 cases; 0%; p<0.0001), while hepatic encephalopathy was not correlated with bare stent use [13.6% (3/22) stent grafts vs. 0% (0/5) bare stents; p=0.19], or larger diameter stent use [9.1% (2/22) 10 mm vs. 10.0% (1/10) >10 mm; p=0.241].
Figure 1
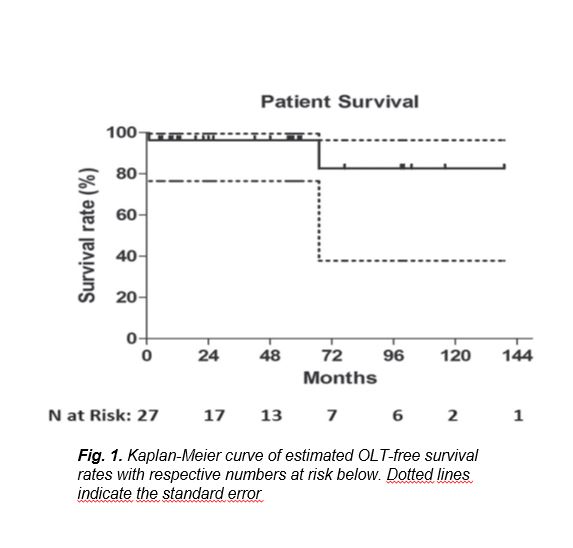
[Figure ID: ]
Figure 2
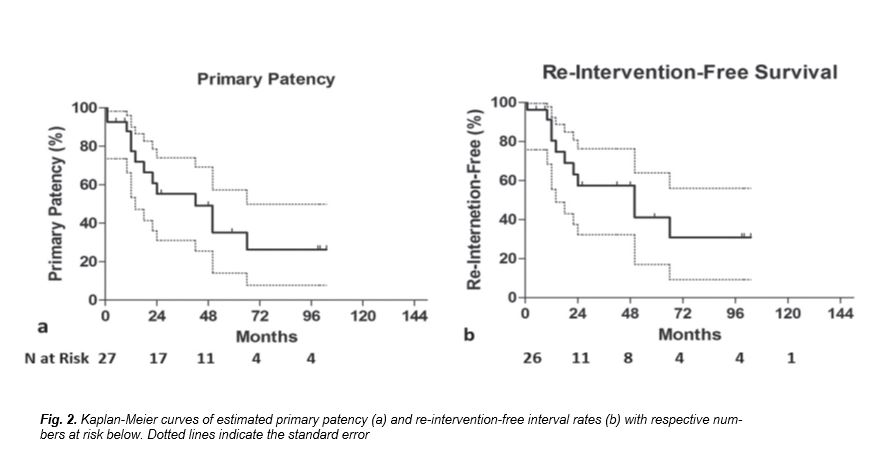
[Figure ID: ]
Figure 3
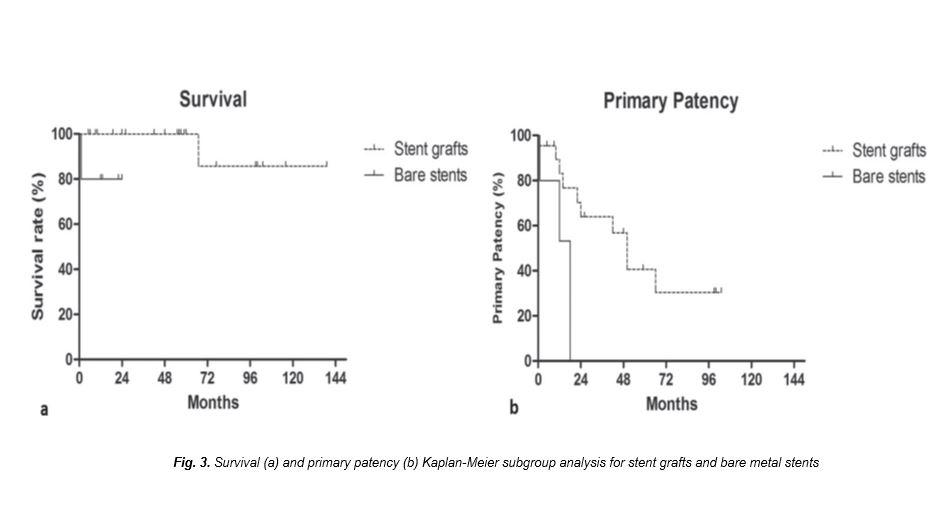
[Figure ID: ]
Discussion
Before 1995 surgical methods represented the main approach following medical treatment failure. In absence of IVC thrombosis, porto-caval shunt is the indicated surgical approach while in case of IVC thrombosis, a cavo-atrial shunt can be considered. In Eastern Asian populations where the hepatic vena cava BCS is the most frequent type, in presence of a membrane occluding the IVC, surgical removal or membrane destruction by angioplasty is the optimal approach [20]. In 1993 Peltzer et al. and Ochs et al. described TIPS technique as a possible treatment in patients with BCS [21, 22] and although OLT may be the only definite therapy, TIPS can significantly improve liver function and can be used as a bridge treatment to OLT; moreover, TIPS is preferable to surgical shunts as it is less invasive and does not affect the anatomy of the IVC, while preserving portal vein patency for future anastomosis [23-26].
In this series, OLT-free survival following TIPS was 96.1%at 5-years and 82.4% up to 10 years follow up. These results are superior to the already satisfactory rates of 74% at 5-years and 69% at 10-years follow up, previously reported in the literature [8, 14]. This difference in survival rates could firstly be attributed to the small number of patients included in all series, which does not allow strong statistical analysis, but also to dissimilar study designs and follow up protocols. In addition, the level of local expertise could also influence outcomes.
TIPS procedure was deemed safe as no procedure-related death occurred and complications rate was in line with literature data [27]. Notably, all bleeding complications were related to percutaneous trans-hepatic liver access, either for targeting the portal vein using a platinum tipped guide wire (in 2 cases) or during the “gun-sight” technique (in 1 case). In the first patient, haemoperitoneum was evident 5 hours after the procedure. Emergency abdominal CT angiography demonstrated active bleeding originating from a right hepatic artery branch which was selectively trans-arterially embolised using micro-coils. In the second patient bleeding was intraoperatively detected. Patient became unstable and as the bleeding site could not be immediately identified, was transferred to theater for laparotomy and haemorrhage was controlled with liver packing. The third patient (“gun-sight” technique) also developed severe hypotension during the procedure. This was attributed to the fact that the targeted portal vein branch was adjacent to the liver capsule, leading to rupture during balloon dilatation. Bleeding was controlled following stent-graft placement. These complications were encountered during initial experience in our center, after which trans-hepatic portal vein access was not further performed. According to the authors’ opinion, trans-hepatic access should be kept as bail out option in cases of inability to gain portal vein access from the internal jugular vein, as it increases the risk of bleeding.
Post-procedural hepatic encephalopathy was encountered in three patients and was successfully treated using standard drug therapy. Clinical success with complete symptoms remission was achieved in all but one case. The only case in which TIPS failed to improve liver function was in a 48-year old female patient suffering from idiopathic BCS, who presented with ascites, MELD score 22 and BSC–TIPS prognostic index of 8.48. This patient died of liver failure one month following the procedure. Both MELD score>18 and BCS-TIPS PI>7 have been previously correlated with decreased survival [28-30]. In this study, all five patients with MELD score>18 demonstrated similar survival rates compared to those with MELD<18. On the other hand as only two patients had BCS-TIPS PI>7, subgroup analysis was not performed. Of note, the second patient with BCS-TIPS PI>7 (7.11) is still alive at 48 months follow up. It should be highlighted that in this patient PI value resulted due to the age of the patient (74 years old) and not to elevated INR (1.54) or bilirubin (1.88 mg/dl) levels, while in the case of clinical failure increased BCS-TIPS PI resulted due to elevated bilirubin (24.2 mg/dl). The authors speculate that severely compromised liver function should negatively influence acute clinical outcome and immediate post-operative survival compared to elevated age. Of note, the prognostic value of BCS-TIPS PI has been recently disputed [31].
According to subgroup analysis, stent type was correlated with increased survival and primary patency, resulting in a mean primary patency time of 50 months vs. 15 months following bare stent deployment. This is in accordance with current literature [32]. In a recent meta-analysis of randomised controlled trials, stent grafts resulted in increased survival (HR=0.67, 95% CI=0.50-0.90) and shunt patency (HR=0.42, 95% CI=0.29-0.62) compared to bare-stents. Moreover, in the same meta-analysis stent grafts were also correlated with decreased rates of hepatic encephalopathy (HR=0.70, 95% CI=0.49-1.00) [33]. In this study, stent grafts were not related to decreased encephalopathy complications. However, this could be due to small sample size resulting in minimal number of events (3 encephalopathy complications).
Finally, five patients remain asymptomatic with patent shunt at 99, 100, 103, 116 and 139 months follow up, respectively, while one of these patients (female; born in 1973) treated with a stent graft, has not undergone any reintervention after 103 months follow up. According to the authors’ experience, supported by herein presented data, achievement of long-term survival was sustained by rigorous clinical, biochemical and imaging follow up, which is of the utmost importance to timely detect disease recurrence and TIPS dysfunction as to preserve shunt patency. In this series reintervention rate was 69.2% at 8 years follow up, while multiple (>1) re-interventions were required in 4 patients (14.8%). Herein presented survival and patency results are in accordance to those recently reported in the literature by Hayek G et al., where TIPS dysfunction in BCS patients, 10-years survival rate was 76%, while cumulative patency rates were 45% [34]. Although bare stents were recognised as a technical factor negatively influencing patency, all re-interventions were performed using stent grafts, so subsequent recurrent shunt occlusion should be attributed to other factors such as non adherence to anticoagulation protocol, underlying disease and patient’s co-morbidities. Unfortunately, this study did not include a sufficient number of patients to perform valid statistical analysis as to clarify factors correlated with recurrent shunt occlusion or the identification of independent predictors of outcomes.
It should be highlighted that reinterventions required due to in-stent restenosis or chronic shunt occlusion can be successfully performed using plain balloon angioplasty and further stenting. On the other hand, acute or subacute stent thrombosis is a more complicated situation which may require the use of combined endovascular techniques in order to safely and effectively remove large thrombus burden and restore shunt patency, such as catheter directed shunt aspiration thrombectomy and/or thrombolysis, rheolytic mechanical thrombectomy and adjunct balloon maceration [26].
Further limitations of this study include the retrospective, single-centre, design, which certainly influences data quality and does not provide external validation, as well as the lack of surgical control group in order to perform treatment comparisons. Moreover, the small number of patients included limits the validity of subgroup analysis. Nonetheless, due to the rarity of the specific pathological entity, as well as the technical difficulty of TIPS creation in BCS, large scale trials are difficult to perform and long-term outcomes from single-centre series remain valuable.
To conclude, TIPS for symptomatic BCS refractory to anticoagulation provides high long-term OLT-free survival rates. Stent grafts were correlated with increased survival and shunt patency and 10 mm stents performed better in terms of patency compared to larger diameter stents. Rigorous follow up and re-interventions due to shunt stenosis/occlusion are imperative to preserve long-term patency and sustain clinical success.
Conflict of interest:
The authors declared no conflicts of interest.
References
1. Parker RGF. Occlusion of the hepatic veins in man. Medicine 1959; 38: 369-402.
2. Budd G. On diseases of the liver. John Churchill, London 1845, pp 135. Brit Lib. 000518193.
3. Chiari H. Erfahrungen über Infarktbildungen in der Leber des Menschen. Zeitschrift für Heilkunde. Prague 1898, pp 475-512.
4. Janssen HL, Garcia-Pagan JC, Elias, E et al. Budd-Chiari syndrome: A review by an expert panel. J Hepatol 2003; 38: 364-371.
5. DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology 2009; 49: 1729-1764.
6. Shin N, Kim YH, Xu H. et al. Redefining Budd-Chiari syndrome: A systematic review. World J Hepatol 2016; 8(16): 691-702.
7. Qi X, Zhang C, Han G, et al. Prevalence of the JAK2V617F mutation in Chinese patients with Budd-Chiari syndrome and portal vein thrombosis: a prospective study. J Gastroenterol Hepatol 2012; 27: 1036-1043.
8. Rossle M, Olschewski M, Siegerstetter V, et al. The Budd-Chiari syndrome: Outcome after treatment with the transjugular intrahepatic portosystemic shunt. Surgery 2004; 135(4): 394-403.
9. Lopez RR, Benner KG, Hall L, et al. Expandable venous stents for treatment of the Budd-Chiari Syndrome. Gastroenterology 1991; 100: 1435-1441.
10. Khuroo MS, Al-Suhabani H, Al-Sebayel M, et al. Budd-Chiari syndrome: Long-term effect on outcome with transjugular intrahepatic portosystemic shunt. J Gastroenterol Hepatol 2005; 20(10): 1494-1502.
11. Plessier A, Sibert A, Consigny Y, et al. Aiming at minimal invasiveness as a therapeutic strategy for Budd– Chiari syndrome. Hepatology 2006; 44: 1308-1316.
12. Bismuth H, Sherlock DJ. Portasystemic shunting versus liver transplantation for the Budd-Chiari syndrome. Ann Surg 1991; 214: 581-589.
13. Henderson JM, Warren WD, Millikan WJ, et al. Surgical options, hematologic evaluation, and pathologic changes in Budd-Chiari syndrome. Am J Surg 1990; 159: 41-50.
14. Garcia-Pagán JC, Heydtmann M, Raffa S, et al. TIPS for Budd-Chiari syndrome: Long-term results and prognostics factors in 124 patients. Gastroenterology 2008; 135(3): 808-815.
15. Ryu RK, DurhamJD, Krysl J, et al. Role of TIPS as a bridge to hepatic transplantation in Budd-Chiari syndrome. J Vasc Interv Radiol 1999; 10(6): 799-805.
16. Fitsiori K, Tsitskari M, Kelekis A, et al. Transjugular Intrahepatic portosystemic shunt for the treatment of Budd-Chiari syndrome patients: Results from a single center. Cardiovasc Intervent Radiol 2014; 37: 691-697.
17. de Franchis R. Evolving consensus in portal hypertension report of the Baveno IV Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43: 167-217.
18. Haskal ZJ, Duszak R Jr, Furth EE. Transjugular intrahepatic transcaval portosystemic shunt: The gun-sight approach. J Vasc Interv Radiol 1996; 7: 139-142.
19. Kanterman RY, Darcy MD, Middleton WD, et al. Doppler sonography findings associated with transjugular intrahepatic portosystemic shunt malfunction. AJR Am J Roentgenol 1997; 168 (2): 467-472.
20. Yamada R, Sato M, Kawabata M, et al. Segmental obstruction of the hepatic inferior vena cava treated by transluminal angioplasty. Radiology 1983; 149: 91-94.
21. Peltzer MY, Ring EJ, LaBerge JM. Treatment of Budd-Chiari syndrome with a transjugular intrahepatic portosystemic shunt. J Vasc Interv Radiol 1993; 4: 263-267.
22. Ochs A, Sellinger M, Haag K, et al. Transjugular intrahepatic portosystemic stent-shunt (TIPS) in the treatment of Budd-Chiari syndrome. J Hepatol 1993; 18: 217-225.
23. Moreno GE, Garcia GI, Gomez SR, et al. Liver transplantation in patients with thrombosis of the portal, splenic or superior mesenteric vein. Br J Surg 1993; 80: 81-85.
24. Attwell A, Ludkowski M, Nash R, et al. Treatment of Budd-Chiari syndrome in a liver transplant unit, the role of transjugular intrahepatic porto-systemic shunt and liver transplantation. Aliment Pharmacol Ther 2004; 20: 867-873.
25. Ganger DR, Klapman JB, McDonald V, et al. Transjugular intrahepatic portosystemic shunt (TIPS) for Budd-Chiari syndrome or portal vein thrombosis. Am J Gastroenterol 1999; 94: 603-608.
26. Tsetis D, Kehagias E, Samonakis D, et al. Percutaneous rheolytic mechanical thrombectomy in thrombosed direct intrahepatic portosystemic shunt: Report of two cases. Interv Med Appl Sci 2015; 7(4): 171-175.
27. Bettinger D, Schultheiss M, Boettler T, et al. Procedural and shunt-related complications and mortality of the transjugular intrahepatic portosystemic shunt (TIPSS). Aliment Pharmacol Ther 2016; 44(10): 1051-1061.
28. Copelan A, Kapoor B, Sands M. Transjugular intrahepatic portosystemic shunt: Indications, contraindications, and patient work-up. Semin Intervent Radiol 2014; 31: 235-242.
29. Salerno F, Merli M, Cazzaniga M, et al. MELD score is better than Child-Pugh score in predicting 3-month survival of patients undergoing transjugular intrahepatic portosystemic shunt. J Hepatol 2002; 36(4): 494-500.
30. Fanelli F. The evolution of transjugular intrahepatic portosystemic shunt: Tips. ISRN Hepatol 2014; 2014: 762096.
31. Rautou PE, Moucari R, Escolano S, et al. Prognostic indices for Budd-Chiari syndrome: Valid for clinical studies but insufficient for individual management. Am J Gastroenterol 2009; 104(5): 1140-1146.
32. Qi X, Yang M, Fan D, et al. Transjugular intrahepatic portosystemic shunt in the treatment of Budd-Chiari syndrome: A critical review of literatures. Scand J Gastroenterol 2013; 48(7): 771-784.
33. Qi X, Tian Y, Zhang W, et al. Covered versus bare stents for transjugular intrahepatic portosystemic shunt: an updated meta-analysis of randomized controlled trials. Therap Adv Gastroenterol 2017; 10(1): 32-41. \
34. Hayek G, Ronot M, Plessier A, et al. Long-term outcome and analysis of dysfunction of transjugular intrahepatic portosystemic shunt placement in chronic primary Budd-Chiari Syndrome. Radiology 2017; 283(1): 280-292.
None
Refbacks
- There are currently no refbacks.







