This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Purpose: To investigate possible associations be¬tween hemodynamic changes and psychoemotion¬al/cognitive status in patients with chronic traumat¬ic brain injury (TBI). Methods and Materials: Dynamic Susceptibility Con¬trast Magnetic Resonance Imaging (DSC MRI) perfu¬sion technique was applied to 22 patients with chronic TBI and 21 healthy volunteers. Patients were divided into moderate/severe and mild TBI groups, accord¬ing to clinical syndromes, and administered episod¬ic memory tests and self-report measures of anxiety and depression symptoms. Cerebral blood flow (CBF) and cerebral blood volume (CBV) values were meas¬ured in normal appearing white matter (NAWM) and normal appearing deep gray matter (NADGM) regions bilaterally, including those involved in episodic mem¬ory and psychoemotional status. Results: The two TBI subgroups differed significant¬ly on episodic memory indices. Significantly reduced CBV and CBF values were detected in the moderate/ severe TBI group compared to controls (p<0.001) in bilateral temporal, right frontal and left pari¬etal NAWM and the semioval center. Perfusion re¬duction in the mild TBI group reached significance, compared to controls, only in the left temporal WM (p<0.002). Substantial negative correlations were found between depression/anxiety scores and CBV values in the mesial temporal lobes (MTL) bilateral¬ly. Mediated regression models indicated that the ef¬fect of reduced CBV in the right MTL on verbal ep¬isodic memory was mediated by increased anxiety symptomatology. Conclusion: Patients with moderate/severe chronic TBI displayed widespread reductions in NAWM CBF and CBV. However, only MTL reduced CBV was as¬sociated with verbal episodic memory deficits and increased psychiatric symptomatology. Mediated regression results were consistent with indirect ef¬fects of reduced CBV on episodic memory capacity through increased anxiety symptoms.
Traumatic brain injury (TBI) is a worldwide problem that results in death and disability for millions of people every year, while over 50% of patients experience chronic neurological deficits and cognitive and functional impairment [1,2]. It is postulated that functional deficits after TBI are partly related to traumatic injury of the neurovascular unit (NVU) -the micro- network that regulates blood flow, vascular permeability and angiogenesis in the central nervous system (CNS) - caused by BBB disruption, edema and focal tissue hypoxia [3-5]. If NVU is not rapidly restored, further local injury is induced [4] with ongoing hypoperfusion and neurodegeneration [5, 6]. These processes apparently take place not only following severe TBI, but, also, after moderate or mild chronic TBI patients [7].
Conventional MRI (i.e., T1SE, T2TSE, FLAIR and GRE sequences), although very sensitive for the detection of TBI lesions, fails to reveal the true extent of structural and functional damage and commonly underestimates Diffuse Axonal Injury (DAI) and atrophy [8]. On the contrary, advanced MRI techniques (i.e, Diffusion Tensor Imaging, perfusion imaging and functional MRI) provide more accurate tools to measure and monitor neurovascular integrity and function, aspiring to improve the understanding of TBI pathophysiology and influence rehabilitation planning in chronic TBI patients [9-12]. In particular, perfusion MRI provides accurate quantitative hemodynamic indices, such as Cerebral Blood Flow (CBF) and Cerebral Blood Volume (CBV), non invasively, and has been proven a valuable tool for both clinical diagnosis and intervention planning [13-16]. Recent Arterial Spin Labeling (ASL) MR perfusion studies have reported widespread reduction in CBF in both normal appearing white matter (NAWM) and normal appearing deep gray matter (NADGM) of acute and chronic TBI patients, indicating diffuse vascular damage and global ischemia [17-23]. Dynamic susceptibility contrast (DSC) MR perfusion imaging allows the assessment of cerebral hemodynamics, by estimating tissue concentration versus time curves after bolus injection of intravascular contrast agents [24-27] and has been used successfully to detect and quantify regional perfusion in acute and chronic TBI [28-30].
An important step in determining the clinical significance of regionally reduced hemodynamic activity entails establishing associations between localized defects on imaging and injury severity, as well as performance on specific cognitive tasks. Published studies directly assessing measures of cerebral blood flow/volume and/ or metabolism and cognitive outcomes in sufficiently large samples of TBI patients are scarce. Wiedmann et al. [31] were among the first to document associations between SPECT-CBF abnormalities in the temporal lobes and memory deficits in 16 chronic TBI patiens. Umile et al. [32] reported a qualitative link between presence of memory deficits and reduced blood flow/metabolism (using PET or SPECT) in the temporal lobes in the subacute phase post mild TBI in 20 patients. Interestingly, several patients demonstrated abnormal findings in PET/SPECT accompanied by cognitive deficits in the absence of structural MRI abnormalities. It should be noted, however, that other studies have failed to identify relations between regional cerebral metabolism (PET) [33] or regional cerebral blood flow (SPECT) [34] and cognitive performance in chronic TBI patients.
CBF measurements in the chronic phase post TBI may serve as an indicator for the mechanism underlying cognitive impairment, as well as for the comprehension of the pattern and degree of neural and/or functional recovery. Sometimes, however, neuroimaging findings are further complicated by failure to take into account frequently occurring psychoemotional difficulties, such as symptoms of depression and anxiety [35]. Such changes may be secondary to physical disability and/or cognitive deficits that affect functionality and post-injury adaptation [36]. An alternative, albeit non-mutually exclusive, account implicates direct effects of neuronal changes in brain regions involved in the generation and/or regulation of emotional states and responses. These regions include the mesial portion of the temporal lobe [37], the dorsolateral prefrontal cortex [38], and the anterior section of the cingulate gyrus [23, 39]. In this context, altered brain function in specific key regions may cause both cognitive and emotional changes, with the latter further impacting the patient’s capacity to perform demanding cognitive tasks [40, 41]. The chief goal of the present preliminary study was to explore the association between hemodynamic disturbances in TBI patients and injury severity, predicting a progressive reduction in CBF and CBV between patients in the chronic phase post mild TBI and chronic moderate/severe TBI patients. A secondary goal was to investigate the functional significance of perfusion changes detected in TBI patients by examining the pattern of associations between perfusion indices and episodic memory capacity, in view of extant data on memory difficulties in chronic TBI patients [42, 43]. Both direct and indirect effects (through psychoemotional variables) of perfusion on episodic memory indices were examined via mediated regression analyses. These models attempted to account for commonly reported, co-occurring cognitive deficits
and psychoemotional difficulties among TBI patients.
Patients with a history of TBI were recruited through the medical records of Neurosurgery Clinic of the University Hospital of Heraklion-Crete. To be included in the study, patients had to be between 20 and 70 years, and have a history of non-penetrating TBI, at least one year before, without neurosurgical intervention. Potential participants were excluded if they had a prior history of pre-morbid neurological or psychiatric disease, current history of substance abuse, or if they were currently receiving psychoactive medications other than anticonvulsants.
The final sample included 22 patients (M/W=20/2), aged
37.6 ± 13.6 years (range 21 to 69) who had sustained TBI on average 31.3 ± 14.4
months ago (range 13 to 72 months) (
Hemodynamic data from 21 healthy controls
(4 men and 17 women, mean age=35.5 ± 6.2 years) were also obtained for
comparison. The study was approved by the University Hospital Ethics Committee
and written informed consent was obtained from all patients, after being
briefed on study details.
Brain MRI examinations were performed on a clinical 1.5 T whole-body superconducting imaging system (Vision/ Sonata, Siemens/Erlangen), equipped with high performance gradients (Gradient strength: 40mT/m, Slew rate: 200mT/m/ms) and a two-element circularly polarizedhead array coil. The conventional imaging protocol was comprised of a 3D T1-w sequence (MPRAGE, TR 1,570/ TE 1.73 ms, 160 axial slices), and contiguous 4 mm thick axial sections of a T2TSE (TR/TE=5,000/98 ms), a TURBO-FLAIR (TR/TE/TI=9,000/120/2,600 ms) and a T2*GRE (TR/T*=615/24 ms) sequence.
The T2* DSC-MRI was performed utilizing a 2D single shot multislice Gradient Echo Echo Planar Imaging (GREEPI) sequence (TR/TE/FA: 1,500 ms/40 ms/30o, BW: 2,442 Hz/pixel, Echo spacing: 0.47 ms and Echo Planar Imaging (EPI) factor 64). Twenty consecutive slices of 4 mm slice thickness and 1.5 mm interslice gap with 50 dynamic acquisitions were obtained. Immediately after the end of the fifth dynamic acquisition, a bolus of 0.1 mmol/kg body weight of gadobutrol (Gadovist, Schering AG, Germany) was injected intravenously, at an injection rate of 4 mL/sec immediately followed by a bolus injection of 15 mL of saline at the same rate. Post processing of the perfusion data was performed using a dedicated software (NordicNeuroLab AS, Bergen, Norway). The arterial input function was calculated by manually defining a major artery (usually MCA) and parametric maps of relative CBV and CBF values were automatically created.
Chronic posttraumatic lesions were identified on T2, FLAIR and GRE sequences and categorized by lobe (frontal, temporal, parietal) and type (contusion or DAI).
CBV and CBF values of NAWM and NADGM areas were calculated in two partially overlapping series of regions. One set comprised 20 sections of the brain including NAWM in the periventricular region, semioval center, forebrain (in the frontal, parietal, temporal, and occipital lobes), splenium and genu of the corpus callosum, and NADGM in the thalami, putamen, and caudate nuclei. In order to enhance measurement fidelity, three CBV and CBF measurements were obtained from each of the different NAWM and NADGM areas, which were then averaged. Two measurements were obtained from each caudate nucleus, due to its small size. All ROIs were fixed in size (radius of 2 mm) and were placed at the bolus peak of the GRE EPI images, which show the vessels to better advantage and thus vascular structures were avoided. From the GREEPI images, ROIs were automatically transferred to the CBV and CBF maps. In order to compare between different subjects, the calculated relative CBV and CBF values were normalized for each patient with respect to the respective values of the cerebellum WM.
A second set of CBV and CBF measurements were obtained in the NAWM of seven sublobar Regions of Interest (ROIs) in each hemisphere, which are suspected to play a key role in the brain circuits responsible for episodic memory and emotional processes, including anxiety and depression: Anterior and mesial temporal lobe (BA 20,36,38), inferior (BA44/45/47), middle (BA46/9), and superior frontal gyri (BA 8/9), inferior parietal lobule (BA 39), and cingulate gyrus (BA32).
Patients were administered a battery of neuropsychological tests primary targeting memory skills. Short-term and working verbal memory was assessed with the Memory for Digits Forward and Reverse subtests, respectively from the Greek Memory Scale (GMS) [45], adapted for research purposes in Greek [46]. Secondary verbal episodic memory was assessed with the GMS Passage Memory subscale and secondary visual episodic memory with the modified Taylor Complex Figure (TCF) test [47]. Normative data were available on a sample of 550 native Greek individuals aged 16-65 years stratified by educational level and geographical origin, permitting computation of age -and education- adjusted standard scores for six subgroups (16-38 and 39-60 years old with 0-9, 10-12 and 13+ years of formal education).
Psychoemotional variables (depression and anxiety self-ratings) were also assessed, using the Greek adaptations of the Center for Epidemiology Studies Depression Scale (CESD) [48] and the Spielberger Trait Anxiety Inventory (STAI-B) [49].
TBI subgroup comparisons on demographic, clinical and neuropsychological data were performed via one-way ANOVAs or Pearson chi-square tests for proportions, when appropriate (evaluated at α=0.05, two tailed). Group differences on normalized, regional perfusion values were assessed using one-way ANOVAs, separately for each brain section and ROI. All tests were evaluated at a Bonferroni-adjusted α=0.05/20 (brain sections)=0.0025, or α=0.05/14 (ROIs)=0.0035, accordingly. Group served as the between subjects variable with three levels: Controls (n=21), Mild TBI (n=8), Moderate/Severe TBI (n=14).
Associations between perfusion measures and clinical, cognitive, or psychoemotional variables were assessed through Pearson correlation coefficients for the entire group of TBI patients. With the exception of anxiety and depression scores all other neuropsychological measures were converted to age -and education- adjusted z scores based on Greek population norms.
Finally, mediated regression models
assessed (a) direct effects of perfusion on episodic memory indices and (b)
indirect effects of psychoemotional status as mediators in the relationship
between perfusion and memory skills. The basic mediated regression model is
illustrated in
Individual scores on the outcome variable Y (raw episodic memory score) were estimated as the sum of the corresponding intercept iy, the direct effect of the perfusion measure on Y controlling for any indirect effects (c’1) and the indirect effect of the perfusion measure on Y according to the following equation:
The multiplicative term bM was computed by multiplying the α and b paths. The program generates bootstrapped confidence intervals for all direct and indirect effects, thus reducing the impact of potential normality violations on significance testing. All statistical analyses were performed with SPSS version 20 (SPSS Inc., Chicago, IL, USA).
| Table 1. Clinical, demographic and neuropsychological information on the patients | |
| N/Mean ± SD | Range |
| Gender Men 20 Women 2 | |
| TBI Severity Mild 8 Moderate 8 Severe 6 | |
| Trauma type MVA 14 Fall 8 | |
| Age (years) 37.6 ± 13.6 | 21 to 69 |
| Education (years) 11.18 ± 4.3 | 1 to 20 |
| Months post injury 31.3 ± 14.4 | 13 to 72 |
| GCS 10.7 ± 3.4 | 3 to 15 |
| Digits Forward (z) -0.18 ± 1.2 | -3.1 to 2.1 |
| Digits Reverse (z) -0.72 ± 1.1** | -3.2 to 0.9 |
| PM-Immediate -1.11 ± 1.0† | -3.1 to 0.7 |
| PM-Delayed -1.4 ± 1.3† | -3.8 to 0.8 |
| PM-Retention -1.6 ± 2.7** | -6.8 to 3.6 |
| PM-Recognition -2.17 ± 2.7† | -6.4 to 1.2 |
| TCF-copy 0.02 ± 1.1 | -1.2 to 1.1 |
| TCF-Memory -0.74 ± 1.3** | -2.9 to 2.2 |
| CESD 20.3 ± 16.2 | 2 to 57 |
| STAI-B (Trait Anxiety) 41.2 ± 13.7 | 21 to 66Abbreviations; GCS: Glasgow Coma Scale, CESD: Center for Epidemiological Studies Depression scale, STAI-A: State-Trait Anxiety Inventory Form Y, GAMA: General Ability Measure for Adults, MVA: Motor Vehicle Accident, TCF: Taylor Complex Figure Test. Note; Significant difference from age -and education- adjusted population mean: ** p=0.01, † p=0.001 |
As shown in Table 1, patients as a group performed significantly below age -and education- adjusted population means on Digits Reverse, Immediate and Delayed Passage recall and recognition, and TCF-Memory scores .
The two TBI severity subgroups differed significantly on episodic memory indices (Passage Memory Immediate recall, F[1,21]=9.13, p=0.007, η2=0.325, Passage Memory Delayed recall, F[1,21]=10.58, p=0.004, η2=0.358, and Passage Memory Recognition, F[1,21]=6.39, p=0.02, η2=0.252). Although patients with moderate/severe TBI had the tendency to report higher frequency of depression and anxiety symptoms than patients following mild TBI, this difference did not reach significance (p>0.13).
Main effects of Group on CBF measured in the 20 NAWM
and NADGM areas meeting the Bonferroni-corrected alpha level of 0.003, were
found in the temporal lobe WM and semioval center bilaterally and also in the
right frontal and left parietal WM (
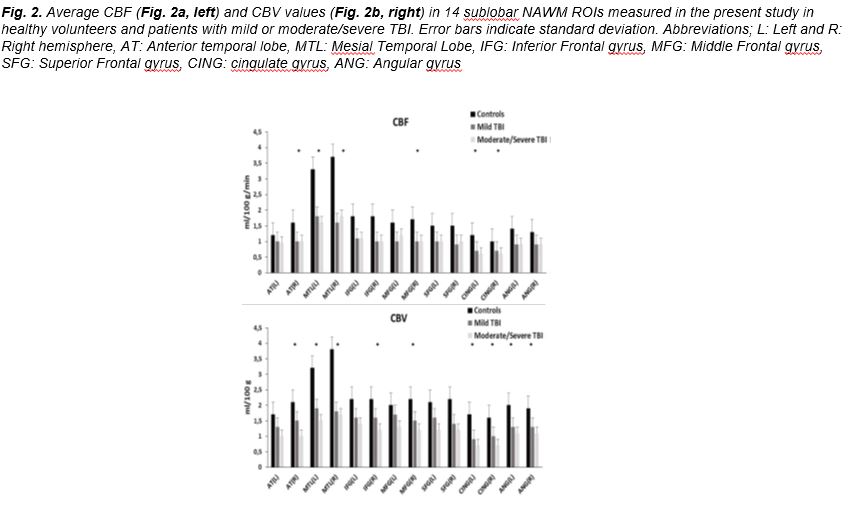
Pearson correlations further suggested that CBF in the left MTL (r=0.500, p=0.021) and nearby ATL (r=0.478, p=0.028) increased with time post injury across TBI subgroups (CBV in these regions and either perfusion measure in predefined WM sections did not correlate significantly with clinical variables). GCS did not correlate significantly (r<0.25) with CBF, CBV or with the type (contusion/DAI) and lobe (temporal/frontal in each hemisphere) of structural abnormalities.
Weak correlations were found among CBV values in the
MTL bilaterally and the MRI-detectable lesions (
Whereas correlations between structural MRI abnormality indices and cognitive variables did not exceed r= -0.25 (p>0.3), significant positive associations were found with psychoemotional status. In particular presence of contusion in the right temporal lobe was a significant predictor of CESD score (r=0.563, p=0.01). The association between right temporal contusion and anxiety did not reach significance (r=0.380, p=0.09).
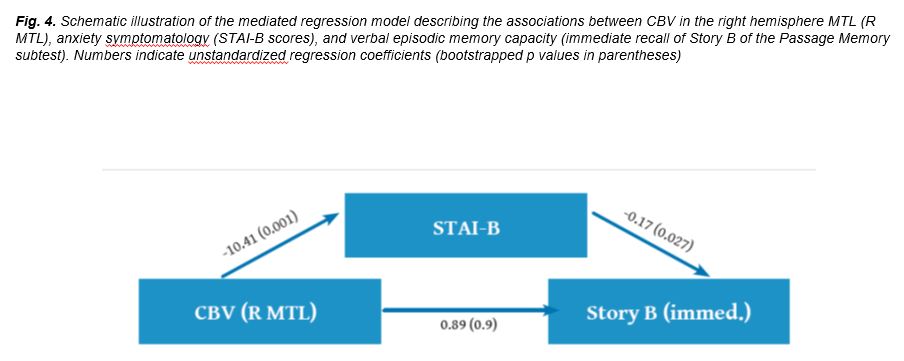
Multiple regression analyses with CESD as the dependent variable, revealed that right MTL CBV (β= -0.527, t= -2.605, p=0.021) and right temporal contusions (β=0.460, t= -2.332, p=0.040) made significant independent contributions to CESD scores (R2=0.402, SE=13.05, p=0.016). Conversely right MTL CBV remained a significant predictor of STAI-B scores after controlling for presence of contusion in the right temporal lobe (R2=0.390, SE=11.33, p=0.019; β= -0.613, t= -3.084, p=0.007), whereas the contribution of contusions controlling for CBV did not reach significance (β=0.05, p>0.8).
Regression models, such as the one presented, in Figure 4 failed to reveal significant mediation (by STAI-B or CESD scores) of the association between structural brain abnormalities and cognitive variables.
Mild TBI patients are less likely to experience cognitive deficits spanning more than one domain. The two groups (mild vs. moderate/severe TBI were largely comparable on the presence of structural abnormalities (cerebral contusion or DAI in the temporal and frontal lobes; p>0.5 for all comparisons).
| Table 2. Individual demographic, clinical, imaging and neuropsychiatric data for TBI patients | ||||||
| Age (years) | Education (years) | GCS | TBI Group | Duration (months) | Lesion | Neuropsychiatric impairment |
| 45 | 17 | 14 | Mild | 22 | RT frontotemporal contusion | Episodic memory (delayed) |
| 42 | 8 | 12 | Moderate | 35 | RT frontotemporal contusion | Episodic memory (immediate/delayed), executive, anxiety, depression |
| 21 | 13 | 13 | Mild | 23 | Bilateral temporal contusion | anxiety, depression |
| 30 | 6 | 10 | Moderate | 48 | RT parietal, bilateral frontal DAI | Episodic memory (immediate/delayed) |
| 58 | 10 | 13 | Moderate | 24 | none | Episodic memory (immediate/delayed) |
| 36 | 13 | 12 | Moderate | 23 | Bilateral frontal contusion, RT frontal DAI | Executive |
| 23 | 10 | 7 | Severe | 26 | Bilateral frontal contusion, RT frontal DAI | Episodic memory (immediate/delayed), anxiety |
| 29 | 11 | 12 | Mild | 15 | Bilateral frontal DAI | Executive |
| 40 | 12 | 14 | Mild | 50 | LT temporal contusion | anxiety |
| 38 | 16 | 7 | Severe | 25 | LT temporo-occipital contusion and DAI, RT temporal and CC DAI | Episodic memory (immediate/delayed) |
| 23 | 12 | 6 | Severe | 18 | LT frontal DAI | Episodic memory (immediate/delayed), executive |
| 48 | 16 | 15 | Mild | 26 | LT temporal & bilateral frontal contusion | Episodic memory (delayed) |
| 40 | 20 | 3 | Severe | 33 | none | none |
| 53 | 1 | 12 | Moderate | 57 | LT frontotemporal contusion, bilateral frontal DAI | Episodic memory (immediate/delayed), executive |
| 29 | 10 | 11 | Moderate | 31 | none | Episodic memory (immediate/delayed), executive |
| 26 | 9 | 4 | Severe | 13 | none | Episodic memory (immediate/delayed), executive |
| 59 | 8 | 13 | Mild | 33 | none | none |
| 22 | 14 | 8 | Severe | 27 | LT Frontoparietal DAI | Episodic memory (immediate) |
| 29 | 13 | 11 | Moderate | 24 | Bilateral frontal & LT temporal DAI | Episodic memory (delayed) |
| 69 | 6 | 13 | Mild | 23 | LT temporal contusion | anxiety |
| 23 | 7 | 13 | Mild | 72 | none | none |
| 44 | 14 | 12 | Moderate | 42 | none | Episodic memory (delayed), executive, depression |
* Significant at Bonferroni-corrected alpha = 0.002. L/R: Left and Right hemispheres, respectively
| Table 3. Perfusion (CBF and CBV) differences between controls and TBI subgroups in NAWM | |||||
| CBF | F | p | η2 | C>Mild | C>Mod/Severe |
| Temporal (L) | 12,657 | 0.000 | 0.388 | 0.001* | 0.0001* |
| Temporal (R) | 6,586 | 0.003 | 0.248 | 0.014 | 0.002* |
| Frontal (R) | 8,310 | 0.001 | 0.294 | 0.005 | 0.001* |
| Parietal (L) | 8,002 | 0.001 | 0.286 | 0.025 | 0.0001* |
| Semioval Center ((L) | 7,861 | 0.001 | 0.282 | 0.006 | 0.001* |
| Semioval Center (R) | 8,428 | 0.001 | 0.296 | 0.003 | 0.001* |
| CBV | F | p | η2 | C>Mild | C>Mod/Severe |
| Temporal (L) | 12,010 | 0.000 | 0.375 | 0.005 | 0.0001* |
| Temporal (R) | 7,334 | 0.002 | 0.268 | 0.02 | 0.001* |
| Frontal (R) | 8,847 | 0.001 | 0.307 | 0.006 | 0.0001* |
| Periventricular (R) | 9,983 | 0.000 | 0.333 | 0.014 | 0.0001* |
* Significant at Bonferroni-corrected alpha =0.002
| Table 4. Perfusion (CBF and CBV) differences between controls and TBI subgroups: ROI analyses | |||||
| CBF | F | p | η2 | C>Mild | C>Mod/Severe |
| ATL (R) | 7.52 | 0.001 | 0.278 | 0.039 | 0.0001* |
| MTL (L) | 8.41 | 0.001 | 0.301 | 0.006 | 0.001* |
| MTL (R) | 10.42 | 0.0001 | 0.348 | 0.035 | 0.001* |
| MFG (R) | 8.86 | 0.001 | 0.313 | 0.043 | 0.0001* |
| Cingulate (L) | 9.87 | 0.0001 | 0.317 | 0.028 | 0.0001* |
| Cingulate (R) | 9.28 | 0.0001 | 0.330 | 0.024 | 0.0001* |
| CBV | F | p | η2 | C>Mild | C>Mod/Severe |
| ATL (R) | 9.77 | 0.0001 | 0.278 | 0.004 | 0.002* |
| MTL (L) | 7.77 | 0.001 | 0.285 | 0.02 | 0.001* |
| MTL (R) | 10.16 | 0.0001 | 0.343 | 0.005 | 0.002* |
| IFG (R) | 12.70 | 0.0001 | 0.394 | 0.002* | 0.0001* |
| MFG (R) | 10.83 | 0.0001 | 0.357 | 0.004 | 0.001* |
| Cingulate (L) | 9.36 | 0.0001 | 0.319 | 0.021 | 0.0001* |
| Cingulate (R) | 7.85 | 0.001 | 0.282 | 0.017 | 0.001* |
| Angular (L) | 8.53 | 0.001 | 0.300 | 0.008 | 0.002* |
| Angular (R) | 6.55 | 0.003 | 0.247 | 0.025 | 0.002* |
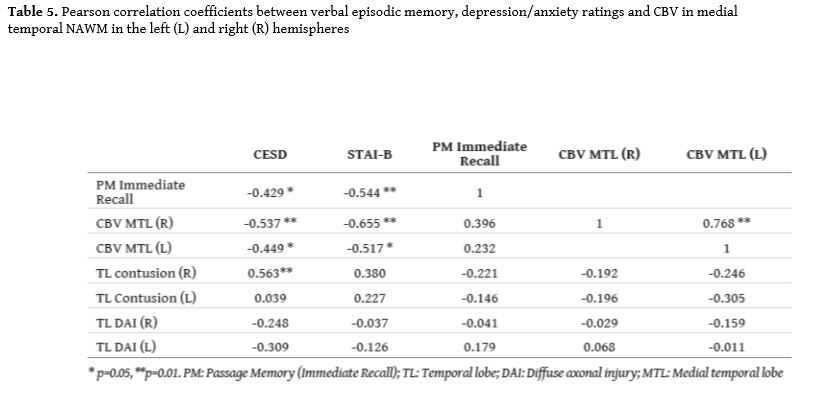
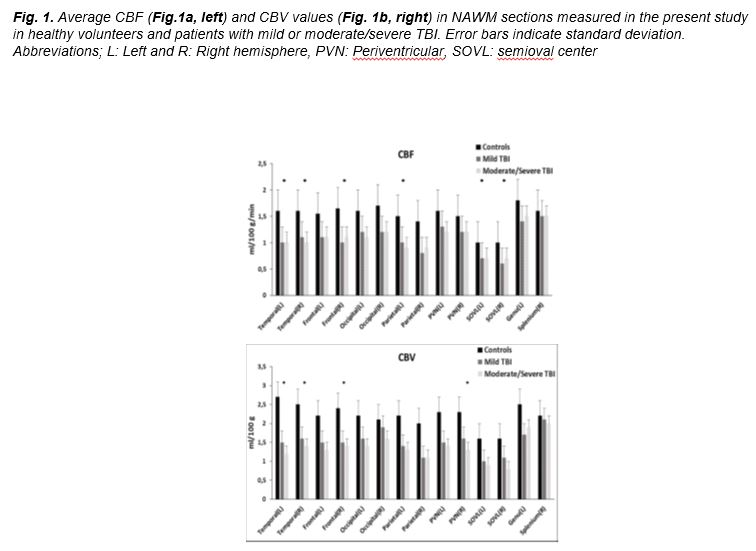
Traumatic forces initiate a cascade of neurovascular responses and perfusion changes that play a significant role in the establishment of chronic TBI structural and functional abnormalities and the development of posttraumatic morbidity [3-5]. Neuroimaging studies have shown hypoperfusion acutely after TBI in humans [7, 17, 34, 50, 51] and experimental TBI in rats [52]. Several investigators have demonstrated cerebral blood flow (CBF) deficits in moderate to/severe TBI, weeks to years after trauma [19,20, 23] while regional hypoperfusion has, also, been reported in chronic mild TBI [18, 21, 22, 30, 53-62 ]. Global or regional hypoperfusion in the chronic phase is probably the result of imbalanced cerebrovascular autoregulation and damaged NYU. There is further evidence of posttraumatic venous damage, even at much lower stretching and shearing forces, that could cause impaired perfusion [63], especially in mild TBI. Our perfusion results showing hypoperfusion in temporal, frontal, periventricular and semioval center WM of moderate/severe TBI patients and in particular temporal and frontal WM regions in the mild TBI group, are in agreement with perfusion abnormalities reported by other neuroimaging techniques. In the current study reduced perfusion was found, additionally, in temporal and frontal regions and the cingulate NAWM bilaterally, regions known to be involved in episodic memory, consistent with previous studies [19-23, 30, 56, 59, 60, 61, 64].
Earlier perfusion studies in chronic TBI, utilizing PET or SPECT, demonstrated several regions of hypoperfusion, particularly in the frontal and temporal lobes, which significantly correlated with neuropsychological or neurological [56-59, 62, 64-67]. Both of these imaging modalities involve radiation exposure and suffer from low spatial resolution, while, PET is, also, costly and difficult to use in clinical practice. ASL MR perfusion imaging studies have revealed similar changes in global and regional CBF in TBI patients across severity subgroups. Patients with chronic moderate-to-severe TBI have decreased regional perfusion in the thalamus, posterior cingulate and frontal cortex, while reduced CBF has also been found in mild TBI, in bilateral frontotemporal regions [18-23, 60].
The DSC MR perfusion technique has been widely used in clinical practice to obtain relative perfusion measurements in a variety of neurological diseases [15, 16, 68] . Its application in TBI patients may significantly contribute to the understanding of the pathophysiology of head injury, and potentially in identifying clinically relevant predictive markers of treatment effects. Using DSC-MRI widespread hypoperfusion has been shown in acute experimental TBI in rats [52] and in acute human moderate to severe TBI cases [29]. Reduced rCBF has also been reported in the cingulated gyrus, cuneus and temporal lobe in chronic mild TBI patients [30] and symptomatic sports-related concussion patients [6]. To our knowledge the current study is the first to report DSC-MRI results in chronic patients over a wide range of TBI severity. A progressive decrease of perfusion between controls, mild, and moderate/severe groups was noted, suggesting chronic microvascular changes that may underlie persistent post-traumatic symptoms or functional abnormalities without apparent neurological deficits.
Associations between reduced perfusion in the left temporal lobe and verbal memory decline found in the present study are consistent with earlier findings establishing links between left temporal damage [69, 70] and reduced regional cerebral blood flow/metabolism [31, 32] with verbal memory deficits in TBI patients. Learning and memory difficulties are among the most common deficits observed in TBI and are often present even following mild TBI [42, 43]. Elevated levels of anxiety and depressive symptomatology are also common in TBI although the causes of such symptoms are still debated [71, 72]. The current data are consistent with a common neurophysiological substrate for both memory and psychiatric symptoms involving dysfunction of circuits comprised to a large extent by medial temporal lobe structures. Thus the left hippocampus and parahippocampal cortex are known to be critically involved in the acquisition of new verbal episodic memories. These structures serve a pivotal role in the consolidation of mnemonic traces [73, 74] through concurrent activity in lateral temporal neocortical regions. These regions are also key components of the medial limbic circuit which is crucial for the generation of emotional responses to external and internal stimuli and in the intrinsic regulation of emotions. Limbic dysfunction has been suggested as one of several neurophysiological correlates of depression [75]. Our findings concur with earlier results in highlighting a direct link between structural damage incurred in right temporal lobe and psychiatric sequelae of TBI (especially depression). In a similar vein, the significant bivariate correlations between bilateral MTL dysfunction (as indicated by reduced CBV) and both depression and anxiety symptomatology are not surprising. To our knowledge the indirect association between reduced left temporal lobe perfusion and verbal memory performance through increased psychiatric symptomatology has not been formally explored before. This model, however, is supported by growing evidence suggesting a causal link between depressive symptomatology and accelerated age-related cognitive decline [76, 77].
Studies establishing links between regional perfusion and psychiatric symptoms are limited. Recently, reduced CBF in the posterior hippocampus was found to be associated with increased depressive symptomatology among patients with chronic heart failure [78]. In TBI, however, the limited thus far investigations implicate structural prefrontal damage in the etiology of depressive symptomatology post TBI [35]. Associations between left temporal structural damage and anxiety symptoms
in chronic TBI have been reported by at least one study [79] consistent with the crucial role of mesial temporal structures (amygdala, hippocampus, parahippocampal gyrus) [80, 81].
The frequency of significant memory deficits among chronic mild TBI patients in our sample is higher than what is typically reported in previous studies and meta-analyses [82]. This could be related to contamination of the mild TBI subgroup by moderate TBI cases, a hypothesis further supported by the relatively high frequency of MR-detectable structural abnormalities among patients with GCS scores consistent with mild TBI.
Notably, measures of immediate verbal episodic recall were significantly related to perfusion measures and depression/anxiety symptoms, whereas delayed recall indices did not. Although the sensitivity of immediate episodic memory indices has been reported in neuropsychological studies of TBI [70, 83], the present results highlight the increased susceptibility of measures of the initial coding and retrieval of verbal information to anxiety and depression in TBI [84] and to a variety of other neuropsychiatric conditions [85, 86, 87]. Stronger links between measures of left MTL integrity and immediate (as opposed to delayed) verbal memory have also been reported in elderly persons with depression [88].
An important limitation of DSC-MRI in clinical practice is the relative rather than absolute quantification of CBF, due to lack of a reliable arterial input function (AIF) [89]. On the contrary, ASL perfusion MRI offers absolute quantification of CBF, without usage of contrast agent, but suffers from a low signal-to-noise ratio and has lower spatial resolution compared with DSC [90]. Similarly to others perfusion techniques, DSC-MRI has limitations in evaluating and interpreting post traumatic hypoperfusion, due to the strong association between CBF measurements and cerebral metabolic demands. Thus, the observed CBF reduction may be the result not only of a primary vascular injury but of neuronal or axonal injury, as well, that reduce cerebral metabolic demand. Recent studies combining MRI with the Blood Oxygen Dependent (BOLD) signal in response to hypercapnia challenge provide more direct measures of cerebral vascular injury by assessing of cerebral vascular reactivity/reverse (CVR) [29]. Finally, this preliminary study has the limitation of the small sample, especially of mild TBI patients and the results should be interpreted with caution. Further perfusion and neuropsychological measurements in a larger number of chronic patients over a wide range of TBI severities may enhance the sensitivity of the method in detecting more subtle and complex associations between perfusion measurements and neuropsychiatric variables.
Despite
the study limitations outlined above, our preliminary results highlighted
robust and widespread reductions in both CBF and CBV in NAWM in the chronic
phase after moderate/severe TBI. Smaller, yet detectable, were CBF/CBF
reductions found among patients with mild TBI, in spite of the small size of
this subgroup. The specificity of the present results is further attested by
the anatomic plausibility of perfusion-behavior associations, identifying
reduced perfusion in the MTL as the sole significant correlate of both verbal
episodic memory deficits and increased psychiatric symptomatology. Finally,
mediated regression analyses are consistent with complex models accounting for
the emergence and parallel course of cognitive and psychoemotional sequelae of
head trauma.
The authors declared no conflicts of interest.
1. Brazinova A, Rehorcikova V, Taylor MS, et al. Epidemiology of traumatic brain injury in Europe: A living systematic review. J Neurotrauma 2018; doi: 10.1089/neu.2015.4126.
2. Thurman DJ, Alverson C, Dunn KA, et al. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil 1999; 14(6): 602-615.
3. Kenney K, Amyot F, Haber M, et al. Cerebral Vascular Injury in Traumatic Brain Injury. Exp Neurol 2016; 275(3): 353-366.
4. Shlosberg D, Benifla M, Kaufer D, et al. Blood-brain barrier breakdown as a therapeutic target in trau
matic brain injury. Nat Rev Neurol 2010; 6(7): 393-403.
5. Del Zoppo GJ .Toward the neurovascular unit. A journey in clinical translation: 2012 Thomas Willis Lecture. Stroke 2013; 44(1): 263-269.
6. Bartnik-Olson BL, Holshouser B, Wang H, et al. Impaired neurovascular unit function contributes to persistent symptoms after concussion: A pilot study. J Neurotrauma 2014; 31(17): 1497-1506.
7. Metting Z, Spikman JM, Rödiger LA, et al. Cerebral perfusion and neuropsychological follow up in mild traumatic brain injury: Acute versus chronic disturbances? Brain Cogn. 2014; 86: 24-31.
8. Rugg-Gunn FJ, Symms MR, Barker GJ, et al. Diffusion imaging shows abnormalities after blunt head trauma when conventional magnetic resonance imaging is normal. J Neurol Neurosurg Psychiatry 2001; 70: 530-533.
9. Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011; 134(2): 449-463.
10. Sharp DJ, Ham TE Investigating white matter injury after mild traumatic brain injury. Curr Opin Neurol 2011; 24(6): 558-563.
11. Wada T, Asano Y, Shinoda J. Decreased fractional anisotropy evaluated using tract-based spatial statistics and correlated with cognitive dysfunction in patients with mild traumatic brain injury in the chronic stage. Am J Neuroradiol 2012; 33(11): 2117-2122.
12. Warner MA, Marquez de la Plata C, Spence J, et al. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J Neurotrauma 2010; 27(12): 2121-2130.
13. Zaharchuk G. Theoritical basis of hemodynamic MR imaging techniques to measure cerebral blood volume, cerebral blood flow, and permeability. Am J Neuroradiol 2007; 28: 1850-1858.
14. Alsop D. Perfusion imaging of the brain. In: Edelman RR, Hesselink JR, Zlatkin MB, Crues JV, editors. Clinical magnetic resonance imaging, 3rd ed. Philadelphia, PA: Saunders Elsevier; 2006. pp 333-376.
15. Pathak AP, Schmainda KM, Ward BD, et al. MR-derived cerebral blood volume maps: Issues regarding histological validation and assessment of tumor angiogenesis. Magn Reson Med 2001; 46: 735-747.
16. Mair G, Wardlaw JM. Imaging of acute stroke prior to treatment: Current practice and evolving techniques.Br J Radiol 2014; 87(1040): 201-216.
17. Doshi H, Wiseman N, Liu J, et al. Cerebral hemodynamic changes of mild traumatic brain injury at the acute stage. PLoS One 2015; 10(2).
18. Ge Y, Patel MB, Chen Q, et al. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labeling MR imaging at 3T. Brain Inj 2009; 23: 666-674.
19. Kim J, Whyte J, Patel S, et al. Resting cerebral blood flow alterations in chronic traumatic brain injury: An arterial spin labeling perfusion fMRI study. J. Neurotrauma 2010; 27(8): 1399-1411.
20. Kim J, Whyte J, Patel S, et al. A Perfusion fMRI Study of the Neural Correlates of Sustained-Attention and Working-Memory Deficits in Chronic Traumatic Brain Injury. Neurorehabilitation and Neural Repair 2012; 26(7): 870-880.
21. Wang Y, West JD, Bailey JN, et al. Decreased cerebral blood flow in chronic pediatric mild TBI: An MRI perfusion study. Dev Neuropsychol 2015; 40(1): 40-44.
22. Grossman EJ, Jensen JH, Babb JS, et al. Cognitive impairment in mild traumatic brain injury: A longitudinal diffusional kurtosis and perfusion imaging study. Am J Neuroradiol 2013; 34(5): 951-957.
23. Newsome MR, Scheibel RS, Chu Z, et al. The relationship of resting cerebral blood flow and brain activation during a social cognition task in adolescents with chronic moderate to severe traumatic brain injury: A preliminary investigation. Int J Dev Neurosci. 2012; 30 (3): 255-266.
24. Rempp KA, Brix G, Wenz F, et al. Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 1994; 193(3): 637-641.
25. Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 1997; 7: 91-101.
26. Ostergaard L, Weisskoff RM, Chesler DA,et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages, part 1: Mathematical approach and statistical analysis. Magn Reson Med 1996; 36: 715-725.
27. Knutsson L, Stahlberg F, Wirestam R. Aspects on the accuracy of cerebral perfusion parameters obtained by dynamic susceptibility contrast MRI: A simulation study. Magn Reson Imaging 2004; 22: 789-798.
28. Garnett MR, Blamire AM, Corkill RG, et al. Abnormal cerebral blood volume in regions of contused and normal appearing brain following traumatic brain
injury using perfusion magnetic resonance imaging. J Neurotrauma 2001; 18(6): 585-593.
29. Kou Z, Ye Y, Haacke EM. Evaluating the Role of Reduced Oxygen Saturation and Vascular Damage in Traumatic Brain Injury Using Magnetic Resonance Perfusion-Weighted Imaging and Susceptibility-Weighted Imaging and Mapping. Top Magn Reson Imaging 2015; 24: 253-265.
30. Liu W, Wang B, Wolfowitz R, et al. Perfusion deficits in patients with mild traumatic brain injury characterized by dynamic susceptibility contrast MRI. NMR Biomed 2013; 26(6): 651-663.
31. Wiedmann KD, Wilson JT, Wyper D, et al. SPECT cerebral blood flow, MR imaging, and neuropsychological findings in traumatic head injury. Neuropsychology 1989; 3: 267-281.
32. Umile EM, Sandel ME, Alavi A, et al. Dynamic imaging in mild traumatic brain injury: Support for the theory of medial temporal vulnerability. Arch Phys Med Rehabil 2002; 83(11): 1506-1513.
33. Kato T, Nakayama N, Yasokawa Y, et al. Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J Neurotrauma 2007; 24(6): 919-926.
34. Hofman PA, Stapert SZ, van Kroonenburgh MJ, et al. MR imaging, single-photon emission CT, and neurocognitive performance after mild traumatic brain injury. Am J Neuroradiol 2001; 22(3): 441-449.
35. Jorge RE, Robinson RG, Starkstein SE, et al. Depression and anxiety following traumatic brain injury. J Neuropsychiat 1993; 5: 369-374.
36. Mooney G, Speed J. The association between mild traumatic brain injury and psychiatric conditions. Brain Inj 2001; 15(10): 865-877.
37. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004; 29(6): 417-426.
38. Koenigs M, Huey ED, Calamia M, et al. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci 2008; 28(47): 12341-12348.
39. Rudebeck PH, Bannerman DM, Rushworth MF. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci. 2008; 8(4): 485-497.
40. Levin HS, Goldstein FC, MacKenzie EJ. Depression as a Secondary Condition Following Mild and Moderate Traumatic Brain Injury. Semin Clin Neuropsychiatry 1997; 2(3): 207-215.
41. Ruff RM, Camenzuli L, Mueller J. Miserable minority: Emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Inj 1996; 10(8): 551-565.
42. Dikmen SS, Machamer JE, Winn HR, et al. Neuropsychological outcome at 1-year post head injury. Neuropsychology 1995; 9(1): 80-90.
43. Lannoo E, Colardyn F, Vandekerckhove T, et al. Subjective complaints versus neuropsychological test performance after moderate to severe head injury. Acta Neurochir Wien 1998; 140(3): 245-253.
44. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2(7872): 81-84.
45. Simos, P G, Papastefanakis E, Panou T, et al. The Greek memory scale. (2011) Rethymno: Laboratory of Applied Psychology, University of Crete
46. Simos P, Ktistaki G, Dimitraki G, et al. Cognitive deficits early in the course of rheumatoid arthritis. J Clin Exp Neuropsychol 2016; 38: 820-829.
47. Hubley AM, Tremblay D. Comparability of total score performance on the Rey- Osterrieth Complex Figure and a modified Taylor Complex Figure. J. Clin. Exp. Neuropsychol 2002; 24: 370-382.
48. Fountoulakis K, Iacovides A, Kleanthous S, et al. Reliability, validity and psychometric properties of the Greek translation of the Center for Epidemiological Studies-Depression (CES-D) Scale. BMC Psychiatry. 2001; 1: 3.
49. Fountoulakis K, Papadopoulou M, Kleanthous S, et al. Reliability and psychometric properties of the Greek translation of the state-trait anxiety inventory form Y: Preliminary data. Ann Gen Psychiatry 2006; 31(5): 2.
50. Menon DK. Brain ischaemia after traumatic brain injury: lessons from 15O2 positron emission tomography. Curr. Opin. Crit Care 2006; 12: 85-89.
51. Rostami E, Engquist H, Enblad P. Imaging of cerebral blood flow in patients with severe traumatic brain injury in the neurointensive care. Front Neurol. 2014; 5: 114.
52. Pasco A, Lemaire L, Franconi F, et al. Perfusional deficit and the dynamics of cerebral edemas in experimental traumatic brain injury using perfusion and diffusion-weighted magnetic resonance imaging. J. Neurotrauma 2007; 24: 1321-1330.
53. Jacobs A, Put E, Ingels M, et al. One-year follow-up of technetium-99m-HMPAO SPECT in mild head injury. J Nucl Med 1996; 37: 1605-1609.
54. Abdel-Dayem HM, Abu-Judeh H, Kumar M, et al. SPECT brain perfusion abnormalities in mild or moderate traumatic brain injury. Clin. Nucl. Med. 1998; 23(5): 309-317.
55. Stamatakis EA, Wilson JT, Hadley DM, et al. SPECT imaging in head injury interpreted with statistical parametric mapping. J. Nucl. Med. 2002; 43(4): 476-483.
56. Gross H, Kling A, Henry G, et al. Local cerebral glucose metabolism in patients with long-term behavioral and cognitive deficits following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 1996; 8(3): 324-334.
57. Barkai G, Goshen E, Tzila ZS, et al. Acetazolamide-enhanced neuro SPECT scan reveals functional impairment after minimal traumatic brain injury not otherwise discernible. Psychiatry Res. 2004; 132: 279-283.
58. Lewine JD, Davis JT, Bigler ED, et al. Objective documentation of traumatic brain injury subsequent to mild head trauma: Multimodal brain imaging with MEG, SPECT, and MRI. J. Head Trauma Rehabil. 2007; 22: 141-155.
59. Bonne O, Gilboa A, LouzounY, et al. Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry Res 2003; 124: 141-152.
60. Kinuya K, Kakuda K, Nobata K, et al. Role of brain perfusion single-photon emission tomography in traumatic head injury. Nucl Med Commun 2004; 25(4): 333-337.
61. Peskind ER, Petrie EC, Cross DJ, et al. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent postconcussive symptoms. Neuroimage 2011; 54(Suppl 1): S76-82.
62. Nakayama N, Okumura A, Shinoda J, et al. Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: An FDGPET study with statistical parametric mapping analysis. J Neurol Neurosurg Psychiatry 2006; 77: 856-862.
63. Haacke WR, Wu B, Kou Z. The presence of venous damage and microbleeds in traumatic brain injury and the potential future role of angiographic and perfusion magnetic resonance imaging. In: Christian W, Kreipke JAR, eds. 2013. Cerebral Blood Flow, Metabolism, Head Trauma.
64. Varney NR, Bushnell DL, Nathan M, et al. NeuroSPECT correlates of disabling mild head injury: Preliminary findings. J Head Trauma Rehabil 1995;10 (3): 18-28.
65. Rao N, Turski PA, Polcyn RE, et al. 18F positron emission computed tomography in closed head injury. Arch Phys Med Rehabil 1984; 65: 780-785.
66. Nakashima T, Nakayama N, Miwa K, et al. Focal brain glucose hypometabolism in patients with neuropsychologic deficits after diffuse axonal injury. Am. J. Neuroradiol 2007; 28(2): 236-242.
67. Raji CA, Tarzwell R, Pavel D, et al. Clinical utility of SPECT neuroimaging in the diagnosis and treatment of traumatic brain injury: A systematic review. PLoS ONE 20149, e91088.
68. Papadaki EZ, Mastorodemos VC, Amanakis EZ, et al. White matter and deep gray matter hemodynamic changes in multiple sclerosis patients with clinically isolated syndrome. Magn Reson Med. 2012; 68(6): 1932-1942.
69. Bigler ED, Blatter DD, Anderson CV, et al. Hippocampal volume in normal aging and traumatic brain injury. Am J Neuroradiol 1997; 18(1): 11-23.
70. Himanen L, Portin R, Isoniemi H, et al. Cognitive functions in relation to MRI findings 30 years after traumatic brain injury. Brain Inj. 2005; 19(2): 93-100.
71. Broshek DK, De Marco AP, Freeman JR. A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 2015; 29(2): 228-237.
72. Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: A meta-analysis. J Neurosurg 2016; 124(2): 511-526.
73. Wang SH, Morris RG. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol 2010; 61:49- 79, C1-4.
74. Ritchey M, Libby LA, Ranganath C. Cortico-hippocampal systems involved in memory and cognition: The PMAT framework. Prog Brain Res. 2015; 219: 45-64.
75. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004; 29(6): 417-426.
76. Köhler S, van Boxtel MP, van Os J, et al. Depressive symptoms and cognitive decline in community-dwelling older adults. J Am Geriatr Soc 2010; 58 (5): 873-879.
77. Panza F, D’Introno A, Colacicco AM, et al. Temporal relationship between depressive symptoms and cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis 2009; 17(4): 899-911.
78. Suzuki H, Matsumoto Y, Ota H, et al. Hippocampal Blood Flow Abnormality Associated With Depressive Symptoms and Cognitive Impairment in Patients With Chronic Heart Failure. Circ J 2016.
79. Knutson KM, Rakowsky ST, Solomon J, et al. Injured brain regions associated with anxiety in Vietnam veterans. Neuropsychologia 2013; 51(4): 686-694.
80. Davidson RJ, Lewis DA, Alloy LB, et al. Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry 2002; 52(6): 478-502.
81. Bishop, S.J. Neurocognitive mechanisms of anxiety: An integrative account. Trends in Cognitive Sciences 2007; 11, 307-316.
82. Belanger HG, Curtiss G, Demery JA, et al. Factors moderating neuropsychological outcomes following mild traumatic brain injury: A meta-analysis. J Int Neuropsychol Soc 2005; 11(3): 215-227.
83. Langeluddecke PM, Lucas SK. WMS-III findings in litigants following moderate to extremely severe brain trauma. J Clin Exp Neuropsychol 2005; 27(5): 576-590.
84. Covassin T, Bay E. Are there gender differences in cognitive function, chronic stress, and neurobehavioral symptoms after mild-to-moderate traumatic brain injury? J Neurosci Nurs 2012; 44(3): 124-133.
85. Faust K, Nelson BD, Sarapas C, et al. Depression and performance on the repeatable battery for the assessment of neuropsychological status. Appl Neuropsychol Adult 2016; 7: 1-7.
86. Johnson LA, Mauer C, Jahn D, et al. Cognitive differences among depressed and non-depressed MCI participants: A project FRONTIER study. Int J Geriatr Psychiatry 2013; 28(4): 377-382.
87. Yoo I, Woo JM, Lee SH, et al. Influence of anxiety symptoms on improvement of neurocognitive functions in patients with major depressive disorder: A 12-week, multicenter, randomized trial of tianeptine versus escitalopram, the CAMPION study. J Affect Disord 2015; 185: 24-30.
88. Avila R, Ribeiz S, Duran FL, et al. Effect of temporal lobe structure volume on memory in elderly depressed patients. Neurobiol Aging 2011; 32(10): 1857-1867.
89. McGehee BE, Pollock JM, Maldjian JA. Brain perfusion imaging: How does it work and what should I use? J Magn Reson Imaging 2012; 36: 1257-1272.
90. Detre JA, Zhang W, Roberts DA, et al. Tissue specific perfusion imaging using arterial spin labeling. NMR Biomed 1994; 7: 75-78.
None







